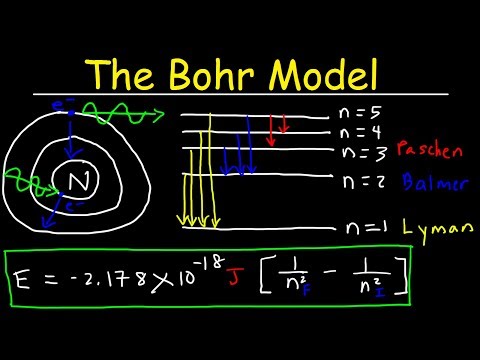
Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed.
Q. Which are older the atoms in the body of your grandmother or those in a new born baby?
Oxygen you breathe today may have been in Texas a few days ago. Which are older, the atoms in the body of your grandmother or those in a new-born baby? They are the same age – most of them nearly that of the universe, as that is when the atoms formed.
Table of Contents
- Q. Which are older the atoms in the body of your grandmother or those in a new born baby?
- Q. Does it make sense to say that a textbook is about 99.9 empty space?
- Q. What does the following element description actually mean Fe 57?
- Q. Why can’t we look at atoms?
- Q. How many electrons are in FE?
- Q. Which is more stable Fe2+ or Fe3+?
- Q. Why is Fe2+ larger than Fe3+?
- Q. What has 53 electrons and 74 neutrons?
- Q. Which element has 16 neutrons?
- Q. What element has 17 protons and 18 neutrons?
- Q. What is the symbol of sulfur?
- Q. What is sulfur mainly used for?
- Q. Is sulfur poisonous to humans?
- Q. Is Sulfur good for the skin?
- Q. What foods are rich in sulfur?
- Q. How much sulfur is in an egg?
- Q. How much sulfur is in a boiled egg?
- Q. Is Sulfur good for hair?
- Q. What pests does sulfur kill?
Q. Does it make sense to say that a textbook is about 99.9 empty space?
Does it make sense to say that a textbook is about 99.9 percent empty space? Yes. A textbook like all material things is made up of atoms, which are considered to be 99.9 percent empty space.
Q. What does the following element description actually mean Fe 57?
the sum of the protons and the neutrons. What does the following element description actually mean? iron-57. iron with a mass number of 57. iron with a sum of the neutrons and the protons equal to 57.
Q. Why can’t we look at atoms?
Atoms are small. In fact, even the most powerful light-focusing microscopes can’t visualise single atoms. What makes an object visible is the way it deflects visible light waves. Atoms are so much smaller than the wavelength of visible light that the two don’t really interact.
Q. How many electrons are in FE?
2, 8, 14, 2
Q. Which is more stable Fe2+ or Fe3+?
Fe3+ is more stable than Fe2+. In Fe3+ ions, there are five 3d half-filled orbitals and is more symmetrical than Fe2+. Whereas in Fe2+ ion there are four 3d half-filled orbitals and one orbital is filled.
Q. Why is Fe2+ larger than Fe3+?
Fe2+ is greater in size as it has lost less electrons than Fe3+ . So, the nuclear pull is more strong on Fe3+ decreasing the atomic size. Thus Fe2+ is greater in size.
Q. What has 53 electrons and 74 neutrons?
iodine
Q. Which element has 16 neutrons?
PHOSPHORUS
Q. What element has 17 protons and 18 neutrons?
chlorine (Cl)
Q. What is the symbol of sulfur?
S
Q. What is sulfur mainly used for?
Elemental sulfur is used in black gunpowder, matches, and fireworks; in the vulcanization of rubber; as a fungicide, insecticide, and fumigant; in the manufacture of phosphate fertilizers; and in the treatment of certain skin diseases. The principal use of sulfur, however, is in the preparation of its compounds.
Q. Is sulfur poisonous to humans?
Sulfur is low in toxicity to people. However, ingesting too much sulfur may cause a burning sensation or diarrhea. Breathing in sulfur dust can irritate the airways or cause coughing. If animals eat too much sulfur, it may be toxic and can be fatal.
Q. Is Sulfur good for the skin?
It is the third most abundant mineral in the human body. Sulfur seems to have antibacterial effects against the bacteria that cause acne. It also might help promote the loosening and shedding of skin. This is believed to help treat skin conditions such as seborrheic dermatitis or acne.
Q. What foods are rich in sulfur?
Foods with Sulfur
- Turkey, beef, eggs, fish, and chicken.
- Nuts, seeds, grains, and legumes.
- Chickpeas, couscous, eggs, lentils, oats, turkey and walnuts.
- Allium Vegetables.
- Cruciferous Vegetables.
- Whole Grains.
- Leafy Green Vegetables.
Q. How much sulfur is in an egg?
Hitchcock’s aversion to eggs may have been influenced by the well-known “rotten egg smell,” usually associated with an egg’s sulfur content (about 50 mg in the white and about 25 mg in the yolk).
Q. How much sulfur is in a boiled egg?
Boiled eggs contain about 180 milligrams sulfur in a 55-gram egg.
Q. Is Sulfur good for hair?
Human hair is made from a protein called keratin, which is high in sulphur content. Sulphur helps to extend the growth phase, ensuring that hair is longer and healthier throughout the cycle, reducing the appearance of thinning hair.
Q. What pests does sulfur kill?
Sulfur is an essential nutrient for plants. Sulfur can kill insects, mites, fungi, and rodents.