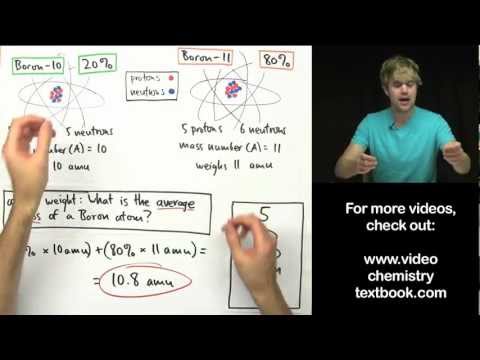
Atomic weight is the weighted average of the naturally occurring isotopes. So again, the mnemonic for memorizing the difference between atomic mass and atomic weight is: atomic mass is the mass of an atom, whereas atomic weight is the weighted average of the naturally occurring isotopes.
Q. What does the atomic weight indicate?
Atomic Mass or Weight Definition Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass indicates the size of an atom.
Table of Contents
- Q. What does the atomic weight indicate?
- Q. Where is the weight of an atom found?
- Q. Do atoms weigh the same?
- Q. Why is atomic mass weighted?
- Q. What is difference between atomic weight and molecular weight?
- Q. How do I calculate molecular weight?
- Q. What is equivalent weight and molecular weight?
- Q. How do you convert atomic weight to molecular weight?
- Q. What is the minimum number of atoms in a molecule?
- Q. What is the difference between an atom or molecule?
- Q. How many atoms are in 1 mole of eggs?
- Q. What lithium looks like atoms?
- Q. What is the formula for li2o?
- Q. Why is li2o ionic?
Q. Where is the weight of an atom found?
Over 99.9 percent of an atom’s mass resides in the nucleus. The protons and neutrons in the center of the atom are about 2,000 times heavier than the electrons orbiting around it. Because the electrons are so light by comparison, they represent only a tiny fraction of a percent of the atom’s total weight.
Q. Do atoms weigh the same?
Atoms of the same chemical element do not always have the same mass because, although the number of protons in the nucleus is the same for all atoms of the same element, the number of neutrons is not. Most elements as they occur naturally on earth are mixtures of several isotopes.
Q. Why is atomic mass weighted?
The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. It is a weighted average because different isotopes have different masses. An atomic mass unit is 1/12th of the mass of a 12C atom.
Q. What is difference between atomic weight and molecular weight?
The molecular weight of a compound is the sum of the atomic weights of the atoms in the molecules that form these compounds. Example: The molecular weight of the sugar molecule found in cane sugar is the sum of the atomic weights of the 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms in a C12H22O11 molecule.
Q. How do I calculate molecular weight?
Sample Molecular Weight Calculation Using the periodic table of the elements to find atomic weights, we find that hydrogen has an atomic weight of 1, and oxygen’s is 16. In order to calculate the molecular weight of one water molecule, we add the contributions from each atom; that is, 2(1) + 1(16) = 18 grams/mole.
Q. What is equivalent weight and molecular weight?
Equivalent weight unlike molecular weight is proportional mass of chemical entities which combine or displace other chemical entities. Equivalent weight. It is defined as the mass of an element/compound/ion which combines or displaces 1 part of hydrogen or 8 parts of oxygen or 35.5 parts of chlorine by mass.
Q. How do you convert atomic weight to molecular weight?
Calculating Molar Mass In 47.88 grams of titanium, there is one mole, or 6.022 x 1023 titanium atoms. The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol).
Q. What is the minimum number of atoms in a molecule?
Molecule: group of two or more atoms held together by chemical bonds. So, minimum 2 atoms are required to form a molecule.
Q. What is the difference between an atom or molecule?
Atoms are single neutral particles. Molecules are neutral particles made of two or more atoms bonded together.
Q. How many atoms are in 1 mole of eggs?
It has the property that 6.022×1023 individual 12C atoms have a mass of 12.00 g precisely. The mole is thus the link between the micro world of atoms and molecules to the macro world of grams and litres.
Q. What lithium looks like atoms?
In the periodic table, it is located in group 1, among the alkali metals. Lithium in its pure form is a soft, silver white metal, that tarnishes and oxidizes very rapidly in air and water….
| General | |
|---|---|
| Appearance | silvery white/grey |
| Atomic properties | |
| Atomic weight | 6.941 amu |
| Atomic radius (calc.) | 145 (167) pm |
Q. What is the formula for li2o?
Li₂O
Q. Why is li2o ionic?
Lithium oxide is an ionic compound formed between a metal (Li) and a non- metal (O) by the complete transfer of electrons from Li to O to give Li+ cations and O2– anions. These ions are held in place in the crystal lattice by strong electrostatic attractions between the positively and negatively charged ions.