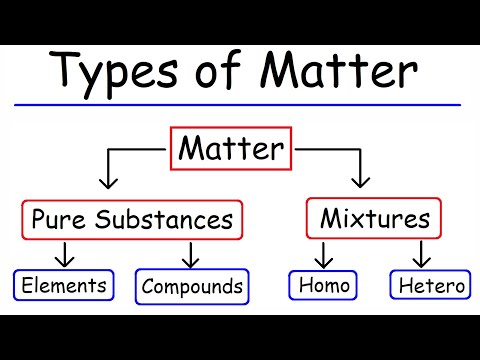
The two main types of pure substances are compounds and elements. They consist of a single type of particle or compound.
Q. What are the 4 classifications of matter?
All “things” are classified as matter. Everything on Earth can be easily described in terms of one of four forms of matter: solid, liquid, gas, and plasma. Students are familiar with the three common forms of matter: solids, liquids, and gases.
Table of Contents
- Q. What are the 4 classifications of matter?
- Q. What are the two 2 classes of matter?
- Q. What element is defined by the following information p += 17 N 20 E -= 17?
- Q. What are the two classes of pure substances?
- Q. What are pure substances Class 9?
- Q. What is the best example of a pure substance?
- Q. What is suspension Class 9?
- Q. What is meant by substance class 9th?
- Q. What is a colloidal solution Class 9?
- Q. What is Tyndall effect class 9?
- Q. Is blood a colloidal solution?
- Q. What is called colloidal solution?
- Q. What are the 5 examples of colloids?
- Q. What are the two types of colloidal solution?
- Q. Is a colloidal solution?
- Q. What are colloidal solutions give example?
- Q. Why milk is a colloidal solution?
- Q. What are the examples of true solution?
- Q. Is apple juice a true solution?
- Q. Is butter a true solution?
- Q. What is difference between true solution and colloids?
- Q. Why is a solution called true solution?
- Q. What are the 5 example of solution?
- Q. Is salt solution is a true solution?
Q. What are the two 2 classes of matter?
Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds.
Q. What element is defined by the following information p += 17 N 20 E -= 17?
Chlorine
Q. What are the two classes of pure substances?
We can divide pure substances into two classes: elements and compounds. Pure substances that cannot be broken down into simpler substances by chemical changes are called elements.
Q. What are pure substances Class 9?
All the elements and compounds are pure substances because they contain only one kind of particles. For Example: Hydrogen,Oxygen,Nitrogen,Chlorine,Iodine,carbon,Iron,Copper etc are pure substances(elements). Water, sodium chloride, Hydrochloric acid,Camphor etc. are pure substances (compounds).
Q. What is the best example of a pure substance?
Examples of pure substances include tin, sulfur, diamond, water, pure sugar (sucrose), table salt (sodium chloride) and baking soda (sodium bicarbonate). Crystals, in general, are pure substances. Tin, sulfur, and diamond are examples of pure substances that are chemical elements. All elements are pure substances.
Q. What is suspension Class 9?
Suspension is the heterogeneous mixture of two or more substances. In suspension, particles are suspended throughout in bulk and can be seen by naked eyes. Example of suspension – mixture of chalk and water, muddy water, mixture of flour and water, mixture of dust particles and air, fog, milk of magnesia, etc.
Q. What is meant by substance class 9th?
Answer: A substance is a matter that has definite properties and composition. Every pure compound and element is a substance.
Q. What is a colloidal solution Class 9?
Class 9 Chemistry Is Matter Around Us Pure. Colloids. Colloids. A colloid is a kind of solution in which the size of solute particles is intermediate between those in true solutions and those in suspensions. For Example: – Soap solution, Milk, Ink, Blood and solutions of synthetic detergents.
Q. What is Tyndall effect class 9?
The Tyndall effect is the phenomenon in which the particles in a colloid scatter the beams of light that are directed at them. This effect is exhibited by all colloidal solutions and some very fine suspensions.
Q. Is blood a colloidal solution?
The process of settling of colloidal particles is called coagulation or precipitation of the sol. Blood is a colloidal solution of an albuminoid substance. The styptic action of alum and ferric chloride solution is due to coagulation of blood forming a clot which stops further bleeding.
Q. What is called colloidal solution?
A mixture in which one substance is divided into minute particles (called colloidal particles) and dispersed throughout a second substance. The mixture is also called a colloidal solution, colloidal system, or colloidal dispersion. The three forms in which all matter exists are solid, liquid or gas.
Q. What are the 5 examples of colloids?
Examples of Colloids
- Colloids refer to dispersions of small particles usually with linear dimensions from around 1 nm to 10 micrometres.
- Examples: fog, smog, and sprays.
- Examples: smoke and dust in the air.
- Examples: milk and mayonnaise.
- Examples: pigmented plastics.
- Examples: silver iodide sol, toothpaste, and Au sol.
- Liquid aerosol.
Q. What are the two types of colloidal solution?
The types of colloids include sol, emulsion, foam, and aerosol. Sol is a colloidal suspension with solid particles in a liquid. An emulsion is between two liquids. Foam is formed when many gas particles are trapped in a liquid or solid.
Q. Is a colloidal solution?
Colloids (also known as colloidal solutions or colloidal systems) are mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance. Colloids usually feature substances that are evenly dispersed in another.
Q. What are colloidal solutions give example?
According to colloidal solution definition, it is defined as a solution in which a material is evenly suspended in a liquid. Some of the Examples of Colloidal Solution are gelatin; muddy water, Butter, blood, Colored Glass.
Q. Why milk is a colloidal solution?
Milk is a colloidal solution because its particles are mixed in such a way that they do not settle at bottom of the container and do not remain unmixed.
Q. What are the examples of true solution?
Answer Expert Verified
- Sucrose dissolved in water.
- Salt solution.
- Lemon juice.
- Urea in water.
- Acetic acid in water.
- Copper sulphate in water.
- All ionic compounds in water.
- Ethanol in water.
Q. Is apple juice a true solution?
Is apple juice a true solution? Even if the apple juice is labelled as ‘100% pure’, it is still a mixture of water particles, sugar particles, flavour particles, and vitamin particles. So it is a mixture, not a pure substance.
Q. Is butter a true solution?
Butter basically is a colloidal solution and hence comes under a type of solution. Butter is basically a solution of water and fat. It is said to be colloidal because the dispersed phase of butter is always a solid by the dispersion medium of butter would be a liquid.
Q. What is difference between true solution and colloids?
True solutions are the type of mixtures, where the solute and solvents are properly mixed in the liquid phase, while Colloidal solutions are the type of mixture in the liquid phase, where the solute (tiny particles or colloids) is uniformly distributed in the solvent (liquid phase).
Q. Why is a solution called true solution?
A solution is called a true solution because a true solution contains all the particles in right composition and correctly dissolved. so a solution is known to be as true solution.
Q. What are the 5 example of solution?
Types of Solution
| S.No | Types of Solution | Examples |
|---|---|---|
| 2 | Solid-liquid | The solution of sugar, salt etc in water. |
| 3 | Solid-gas | Sublimation of substances like iodine, camphor etc into the air. |
| 4 | Liquid-solid | Hydrated salts, mercury in amalgamated zinc, etc. |
| 5 | Liquid-liquid | Alcohol in water, benzene in toluene |
Q. Is salt solution is a true solution?
Salt water is a true solution because it dissolves completely in water and it makes a homogeneous mixture .