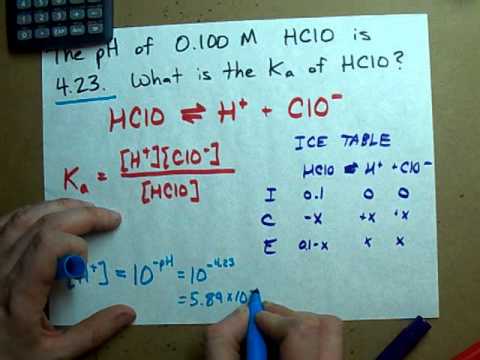The Ka expression is Ka = [H3O+][C2H3O2-] / [HC2H3O2]. The problem provided us with a few bits of information: that the acetic acid concentration is 0.9 M, and its hydronium ion concentration is 4 * 10^-3 M. Since the equation is in equilibrium, the H3O+ concentration is equal to the C2H3O2- concentration.
Q. Does a higher ka mean stronger acid?
Strong acids have exceptionally high Ka values. The higher the Ka, the more the acid dissociates. Thus, strong acids must dissociate more in water. In contrast, a weak acid is less likely to ionize and release a hydrogen ion, thus resulting in a less acidic solution.
Table of Contents
Q. Which Ka is the strongest acid?
hydrochloric acid
Q. What Ka values represents the weakest acid?
The smaller the number the weaker it is. If Ka > 0 it is a strong acid, if Ka < 0 it is a weak acid.
Q. What is the Ka of weak acids?
Ka is the equilibrium constant for the dissociation reaction of a weak acid. A weak acid is one that only partially dissociates in water or an aqueous solution. The value of Ka is used to calculate the pH of weak acids….
| Ka of Weak Acids | ascorbic (I) |
|---|---|
| H 2C 6H 6O 6 | |
| 7.9 x 10 -5 | |
| 4.1 |
Q. Which is the strongest acid pKa value is given?
3.77
Q. Does protonation increase pH?
That is too high to build up a significant amount of the deprotonated species in water, which has a pKa (in water) of 14. As a result of these structural changes at different pH, proteins can change protonation states when the pH changes….2.4: The Effect of pH.
| [H3O+] (mol L-1) | pH |
|---|---|
| 0.1 | 1 |
| 0.01 | 2 |
| 0.001 | 3 |
| 0.0001 | 4 |
Q. What would 7 pH indicate?
pH is a measure of how acidic/basic water is. The range goes from 0 to 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic.
Q. How does pKa affect pH?
Relative Acidity and pKa Values. An application of the Henderson-Hasselbach Equation is the ability to determine the relative acidity of compounds by comparing their pKa values. The stronger an acid, the greater the ionization, the lower the pKa, and the lower the pH the compound will produce in solution.