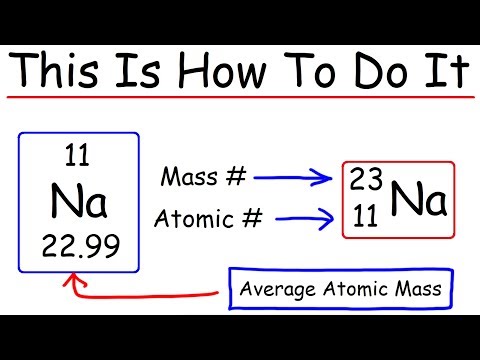
To calculate the numbers of subatomic particles in an atom use its atomic number and mass number:
Q. How do you find the atomic number and protons and neutrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
Table of Contents
- Q. How do you find the atomic number and protons and neutrons?
- Q. Does atomic number equal electrons?
- Q. What is cation give an example?
- Q. Is Potassium a cation or anion?
- Q. Is oxygen a cation or anion?
- Q. Is Sulfur positive or negative?
- Q. Is N3 a cation or anion?
- Q. Is iodine anion or cation?
- Q. Why iodine is not soluble in water?
- Q. Is iodine solution acidic or basic?
- Q. Does iodine change color in water?
- number of protons = atomic number.
- number of electrons = atomic number.
- number of neutrons = mass number – atomic number.
Q. Does atomic number equal electrons?
The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom.
Q. What is cation give an example?
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Some examples of cations are Calcium (Ca2+), Potassium (K+), hydrogen (H+).
Q. Is Potassium a cation or anion?
It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation. Potassium is the major cation (positive ion) inside animal cells, while sodium is the major cation outside animal cells….4.3Related Element.
| Element Name | Potassium |
|---|---|
| Atomic Number | 19 |
Q. Is oxygen a cation or anion?
Because there is no charge, oxygen is neither a cation or an anion. About 20% of air is oxygen, and oxygen is very reactive. In addition to the pure oxygen in air and the pure oxygen dissolved in water, there are many compounds that contain oxygen.
Q. Is Sulfur positive or negative?
Sulfur is in group 6 of the periodic table. What is the charge on its ions, and is the charge positive or negative? The charge is negative, since sulfur is a non-metal. The charge on the ion is (8 – 6) = 2.
Q. Is N3 a cation or anion?
The ion that has the positive charge is called a cation, while the ion which has the negative charge on it is called as an anion….Comparison Chart.
| Basis for Comparison | Cation | Anion |
|---|---|---|
| Examples | Iron (Fe2+), Sodium (Na+), Lead (Pb2+). | Fluoride (F-), Bromide (Br-), Iodide (I-), Nitride (N3-) and Hydride (H-). |
Q. Is iodine anion or cation?
Iodide is a halide anion and a monoatomic iodine. It has a role as a human metabolite. It is a conjugate base of a hydrogen iodide….4.3Related Element.
| Element Name | Iodine |
|---|---|
| Atomic Number | 53 |
Q. Why iodine is not soluble in water?
Non-polar Iodine is not very soluble in water. An intermolecular bond between an induced dipole (I2) and a polar bond in water is not very strong compared to the hydrogen bonds in water. The water molecules would rather remain hydrogen bonded to each other, then to allow an iodine molecule come between them.
Q. Is iodine solution acidic or basic?
Iodine is neither an acid NOR a base…..
Q. Does iodine change color in water?
A solution of iodine (I2) and potassium iodide (KI) in water has a light orange-brown color. If it is added to a sample that contains starch, such as the bread pictured above, the color changes to a deep blue.